Clinical Trials 101

Written By: Shannon Benson, Director of Clinical Trials Program, Mission Cancer + Blood
The Iowa Cancer Plan, our state’s blueprint for cancer control, contains a variety of action items aimed at increasing awareness and participation in cancer clinical trials. But, what exactly is a clinical trial?
Overview
Clinical trials are important in developing new treatments for serious diseases like cancer, because they are research studies to test how well new medical approaches work in people (ex. cancer drugs, therapies, or medical devices). These scientific discoveries provide insight into cancer development and can lead to improved cancer treatments.
It is important to recognize that in past decades, clinical trials were often thought of as a patient’s “last resort” – but this is not true today. In fact, nearly all of today’s most effective cancer treatments are based on the results of previous clinical trials!
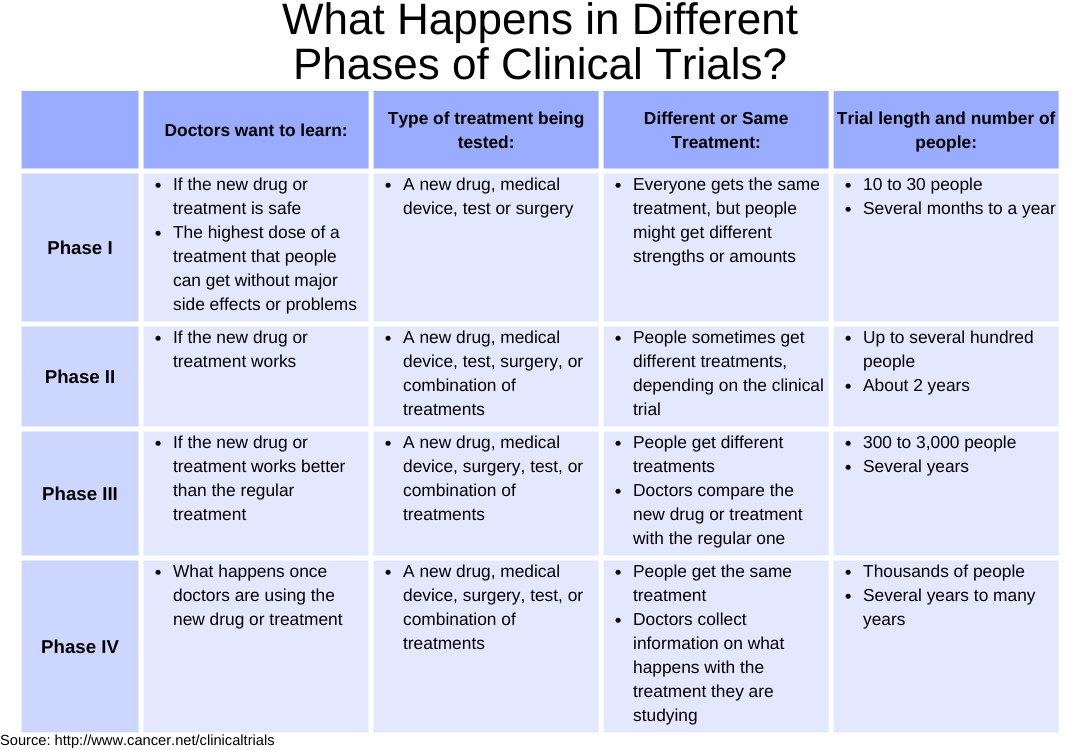
Cancer clinical trials are categorized into four distinct phases:
Participation
Patient participation in clinical trials is critical.
As an example, an estimated 60% of children with cancer in the United States participate in clinical trials. Which has allowed for great improvements in treating pediatric cancer. In contrast, less than 5% of adults with cancer participate in clinical trials. Possible barriers to accessing clinical trials include:
- Lack of awareness: patients may not know a clinical trial may exist.
- Lack of access: not all cancer centers offer or participate in clinical trials.
- Feelings of fear and mistrust: patients may not be able to choose which treatment arm of the trial they enroll on, or they may have a fear that they won’t receive treatment.
- Personal obstacles: a patient may have to travel to a location that offers a clinical trial.
- Insurance Coverage: uncertainty about insurance coverage for the trial and out-of-pocket costs.
Safety
To ensure safety, all clinical trials have and follow a protocol. Their protocol clearly indicates the reason for doing the study, who can join, how many participants are needed, what the treatment will be, what medical testing will be done and how information will be gathered. Each clinical trial has strict eligibility criteria so that data is accurate and meaningful.
Participant protection is a priority with clinical trials. All patients that enroll in a clinical trial provide informed consent. Trials are overseen by scientific review panels, institutional review boards, as well as data and safety monitoring boards.
Note: All new treatments must go through clinical trials before being approved by the Food and Drug Administration (FDA). Cancer clinical trials can take years to complete. It can take months, if not years, to see if a cancer treatment does what it is meant to do.
Looking to the Future
People often hear about the results of clinical trials from overly positive or overly negative media reports. These can influence the way people think about clinical trials and can reinforce common myths. It’s important for those of us in the cancer field to spread the word: clinical trials offer best thinking toward finding better ways to prevent, treat and cure cancer and should be offered to all patients and their families for consideration.
Find a Cancer Clinical Trial in Iowa
Visit the Iowa Cancer Consortium’s Cancer Clinical Trials in Iowa page to search for clinical trials available to you and your loved ones.